1 A is defined as the flow of 1 Cs past a given point 1 C 1 As. ΔG ΔH - TΔS ΔG change in Gibbs free energy for the reaction kJ mol -1.

Gibbs Free Energy Dg Dh Tds Chad S Prep
Gibbs free energy equation.

Gibbs free energy units. The resulting electric current is measured in coulombs C an SI unit that measures the number of electrons passing a given point in 1 s. The change in the Gibbs free energy of the system that occurs during a reaction is therefore equal to the change in the enthalpy of the system minus the change in the product of the temperature times the entropy of the system. Free energy or Gibbs function is by definition g h - Ts where h is enthalpy Jkmol T is absolute temperature K and s is entropy Jkmol.
The Gibbs Free Energy of Formation for enstatite from oxides MgO and SiO 2 Δ G f enstatite oxides is about -354 Jmole at room temperature and pressure. Gibbs free energy G is defined as G H - TS where H T and S are the enthalpy temperature and entropy. Gibbs Energy values are most often today given in units of joulesmole or less commonly caloriesmole.
The Gibbs free energy SI units kJmol is the maximum amount of non-expansion work that can be extracted from a thermodynamically closed system one that can exchange heat and work with its surroundings but not matter. The equation is given as. If the value of Gibbs free energy is in the.
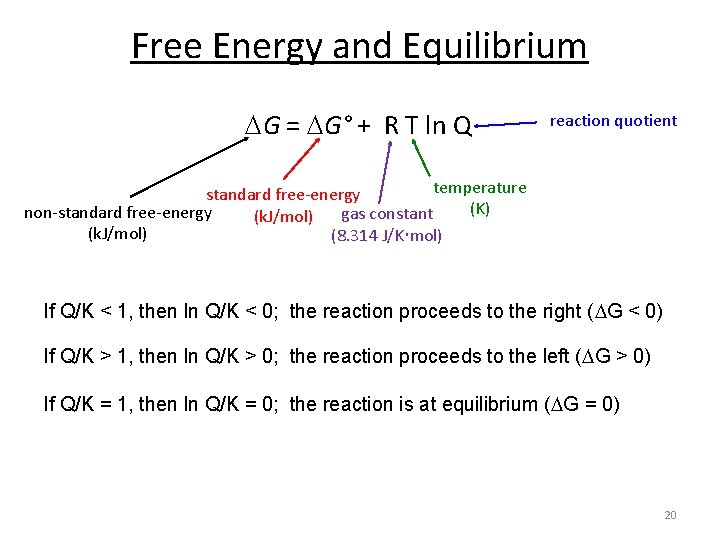
Electric current is measured in amperes A. When a system changes from an initial state to a final state the Gibbs free energy ΔG equals the work exchanged by the system with its surroundings minus the work of the pressure force. G H - TDS The free energy change DG is equal to -TDSuniv and it applies just to a system itself without regard for the surroundings.
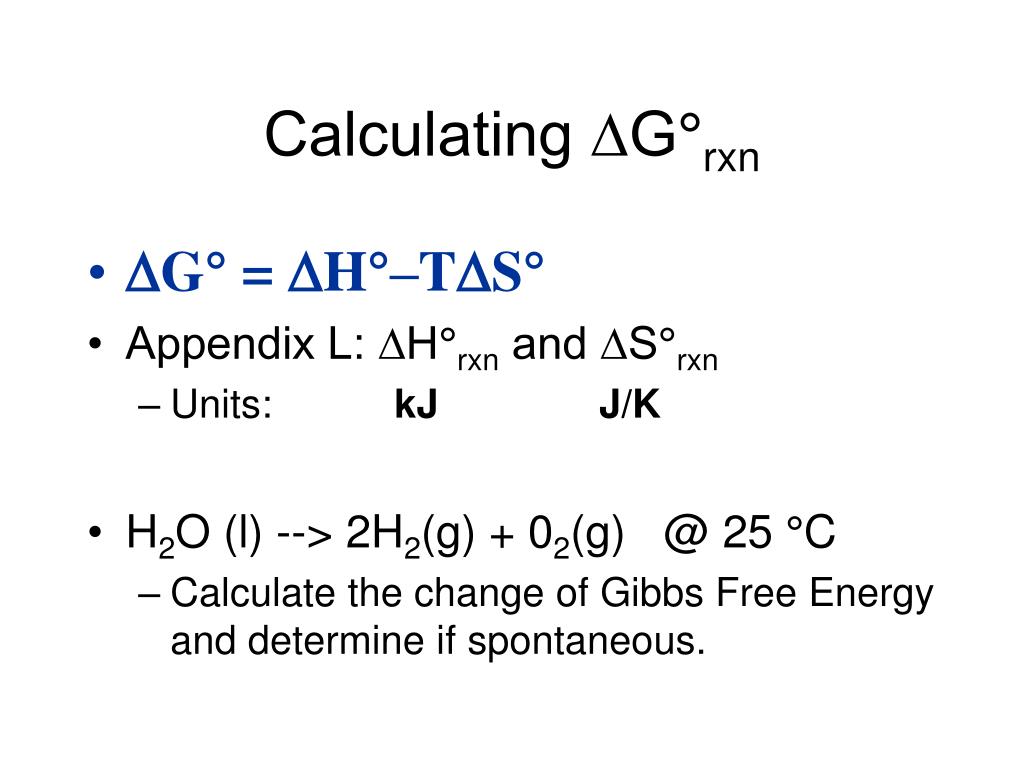
G H TS. A much more serious difficulty with the Gibbs function particularly in the context of chemistry is that although G has the units of energy joules or in its intensive form J mol 1 it lacks one of the most important attributes of energy in that it is not conserved. G H - TS If the reaction is run at constant temperature this equation can be written as follows.
Standard or absolute enthalpy is defined as that based on a reference wherein the value is zero for the elemental substances. The SI unit for Gibbs energy is the kilojoule. Therefore the units of Gibbs free energy are the units of energy such as joule J and calorie cal.
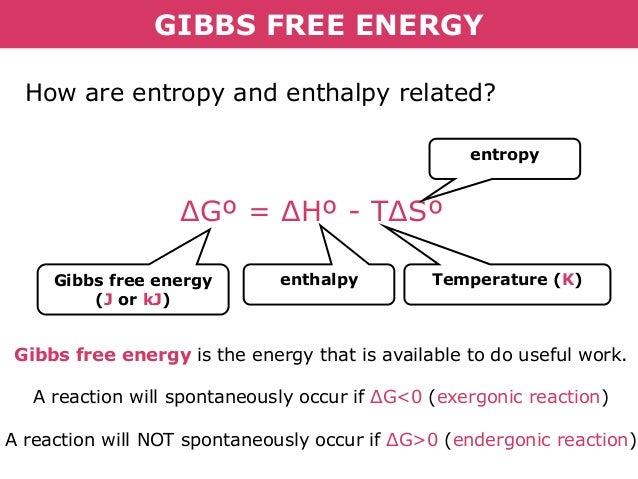
Moreover this potential is used to calculate the optimum of reversible work that one thermodynamic system can perform at constant pressure and temperature. G H - TS. Gibbs free energy G usually has the units kilojoules per mole kJ mol -1 The change in Gibbs free energy ΔG for a chemical reaction at constant temperature T and pressure can be calculated.
So change in Gibbs free energy is equal to the change in. A coulomb relates energy in joules to electrical potential in volts. Gibbs free energy is equal to the enthalpy of the system minus the product of the temperature and entropy.
Gibbs Free Energy Equation. Again by dimensional analysis Helmholtz free energy also has the SI units of Joule. Free Energy is not energy.
ΔG ΔH - TΔS ΔG -8904 - 298-02442 -8176 kJ mol-1 It is easy as long as you remember to convert the entropy change value into kJ. A U T S. Hence by dimensional analysis Gibbs free energy has the SI units of Joule.
Gibbs free energy is measured in Joules according to the International System in ergs for the Cegesimal System of Units in calories or in electron volts for electro Volts. Thus although the free energy always falls when a gas expands or a chemical reaction takes place. The Gibbs free energy equation is dependent on pressure.
The Gibbs free energy commonly called G is a thermodynamic potential defined as the difference of the enthalpy H minus the product of the temperature T by the entropy S of the system. So if you had to calculate the Gibbs free energy change at say 298 K you can just slot the numbers in. This maximum can be attained only in a completely reversible process.
The Δ G f values given above for enstatite are both negative. Gibbs free energy is a state function hence it doesnt depend on the path. The SI unit of Gibbs free energy is joule J.
It is defined by the Gibbs equation. Free Energy and Free Energy Changethe Gibbs free energy G is used to describe the spontaneity of a process. Since both are only free energies it makes sense to assign them the SI unit Joule.
G H - T S. In thermodynamics Gibbs free energy is known as a thermodynamic potential. Where G Gibbs free energy.

Gibbs Free Energy Example Video Khan Academy
Ppt Gibbs Free Energy Powerpoint Presentation Free Download Id 4783967

Delta G Is Energy Availabe To Do Useful Work And Is Thus A Meaure
Chapter 18 Entropy Free Energy And Equilibrium Ppt Video Online Download

Unit 11 Chp 5 8 19 Thermodynamics H S G K Ppt Video Online Download

Gibbs Free Energy Boundless Chemistry
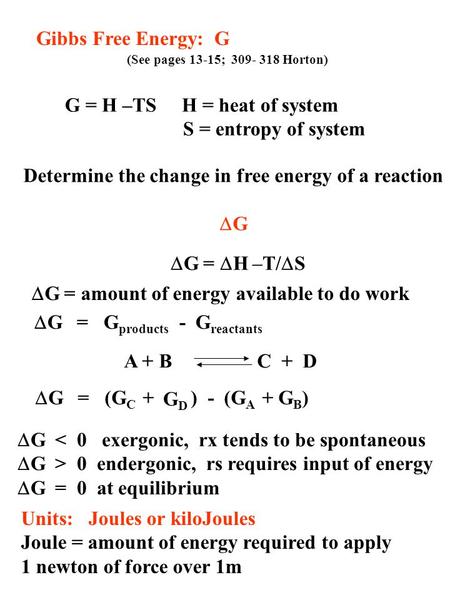
Gibbs Free Energy G See Pages 13 15
Cell Potential Gibbs Free Energy Standard Reduction Potentials Electrochemistry Problems Youtube

Chapter 2 The First Law Of Thermodynamics For Closed Systems Thermodynamics

Physical Chemistry Of Polymers Powerpoint Slides

Excess Gibbs Free Energy Youtube
Gibbs Free Energy Gibbs Free Energy Enthalpy

The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com
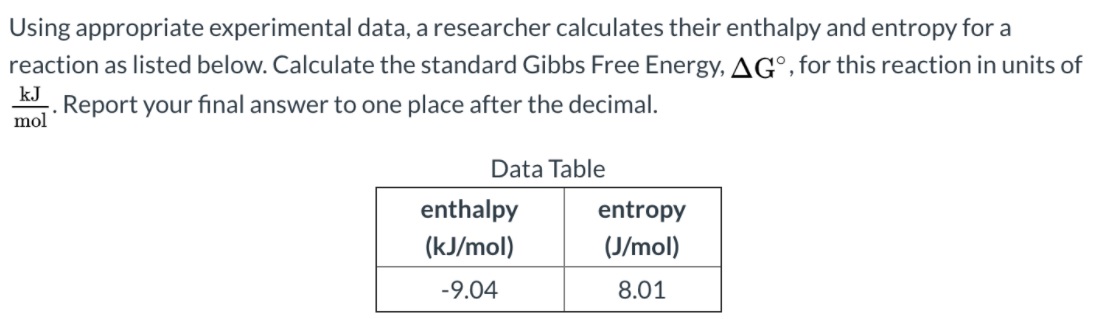
Answered Using Appropriate Experimental Data A Bartleby

Dpy2 Unit 5 Lesson 4 Spontaneity Gibbs Free Energy Equilibrium Lessons Blendspace
How Is Gibbs Free Energy Related To Enthalpy And Entropy Socratic

Lecture 16 Thermodynamics Gibbs Free Energy And Entropy Unit Iii Thermodynamics Chemical Equilibrium Principles Of Chemical Science Chemistry Mit Opencourseware
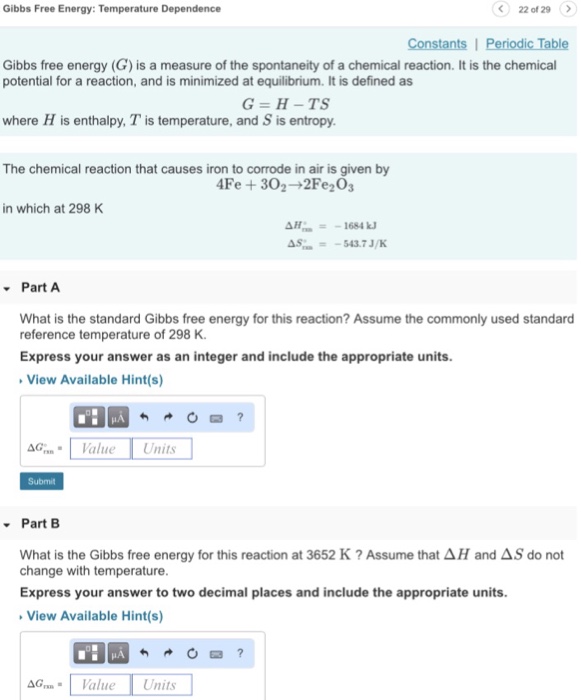
Solved Gibbs Free Energy Temperature Dependence 22 Of 29 Chegg Com
Affinity Association Constant And Dissociation Constant Deranged Physiology
Chapter 17 1 Gibbs Free Energy For A
O Millesing 2 50 Pts In Chemistry 325 You Will Learn Or Learned That The Standard Gibbs Free Energy Change For A Chemical Reaction Is Course Hero
Tang 01b Enthalpy Entropy And Gibb S Free Energy

Ak Lectures Cell Voltage And Gibbs Free Energy

Atp Hydrolysis Gibbs Free Energy Video Khan Academy
Chemical Potential And Gibbs Free Energy Mrs Bulletin Cambridge Core

15 2 Gibbs Free Energy Hl Youtube

Gibbs Free Energy Of Activation An Overview Sciencedirect Topics

Standard Free Energy Changes Introduction To Chemistry

Derivation Of Gibbs Free Energy Formula Chemistry Stack Exchange

New Chm 152 Unit 6 Power Points Sp13

The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com

Free Energy Delta G And Equilibrium Pt 8 Youtube
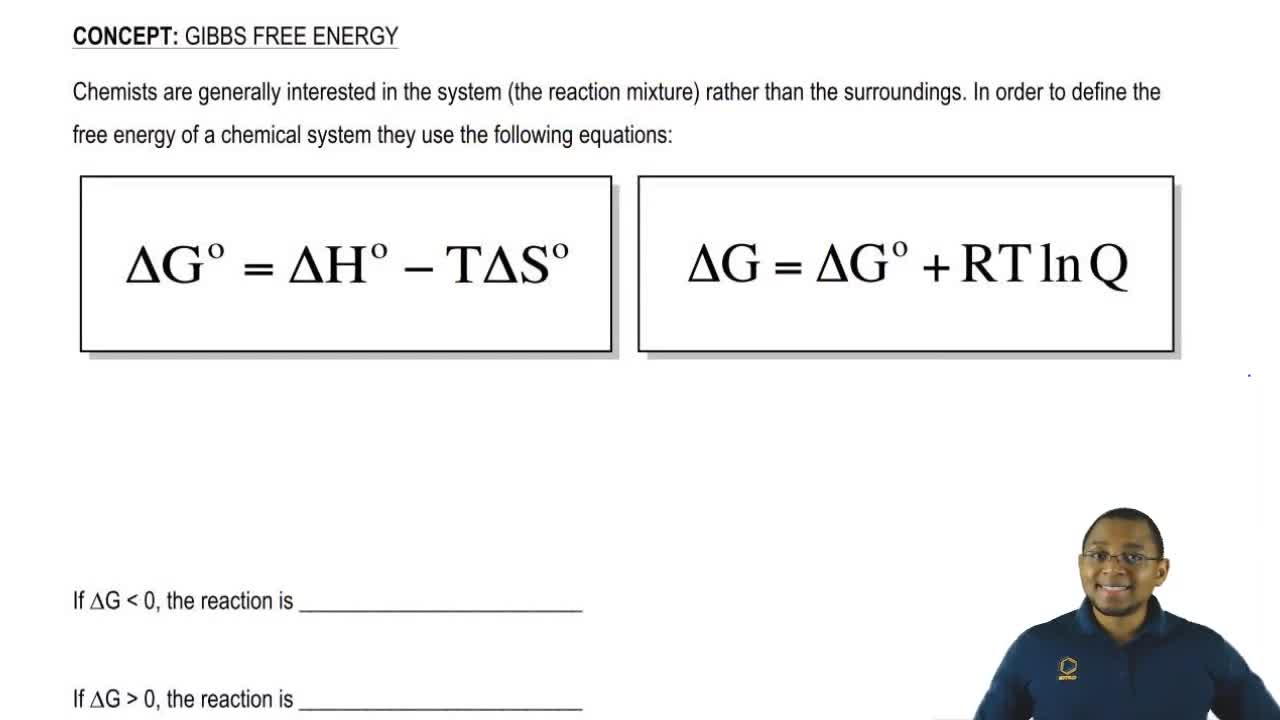
Gibbs Free Energy Chemistry Video Clutch Prep

Unit 16 Enthalpy Entropy And Free Energy Voorhees Science

Gibbs Free Energy Of Important Reactions Involved In Nitrogen Download Table
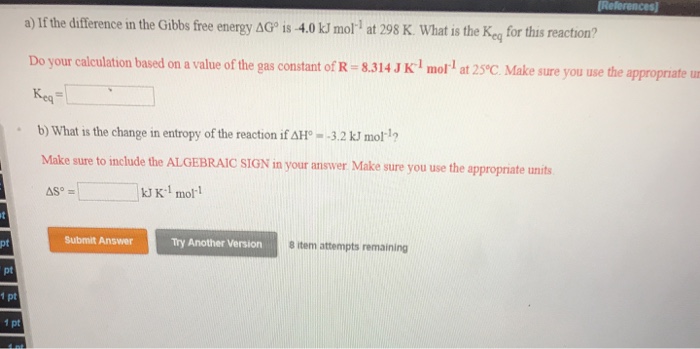
Solved A If The Difference In The Gibbs Free Energy Dgo Chegg Com

Standard Gibbs Free Energy Changes Ag At 25 And 55 C For Reactions Download Table

Gibbs Free Energy And Chemical Potential Nc State University Pdf Free Download
Chapter 13 Principles Of Bioenergetics

Gibbs Free Energy Of Mixing Youtube

Free Energy And Pressure Concentration Ap Chemistry

Gibbs Free Energy Boundless Chemistry

New Chm 152 Unit 6 Power Points Sp13
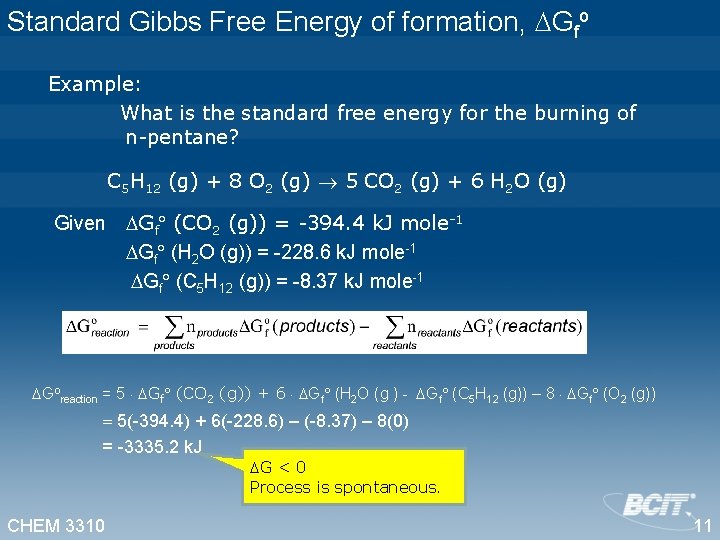
Chem 3310 Thermodynamics Change In Gibbs Free Energy
Hft 2 Above Is A Mock Human Free Energy Table Showing The Thermodynamic Data Quantities For A Selection Of 13 Hypothetical People Wherein The Names Of The Organic Substances From Raymond Chang S 1998 Thermodynamic Data Table Nº Have Been

Gibbs Free Energy Temperature And Spontaneity Ppt Download

Free Energy Metabolism Metabolic Pathways Flashcards Quizlet
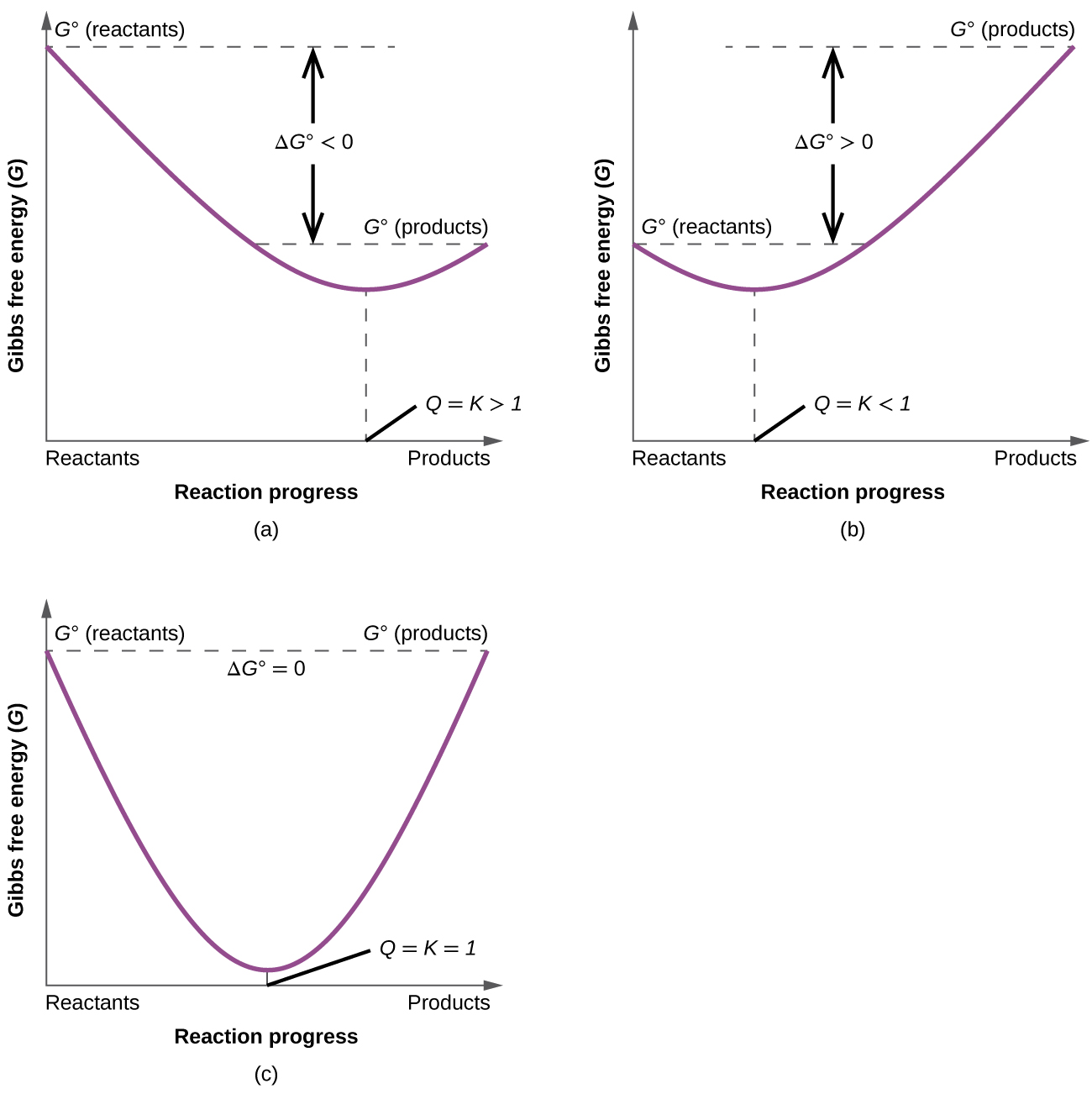
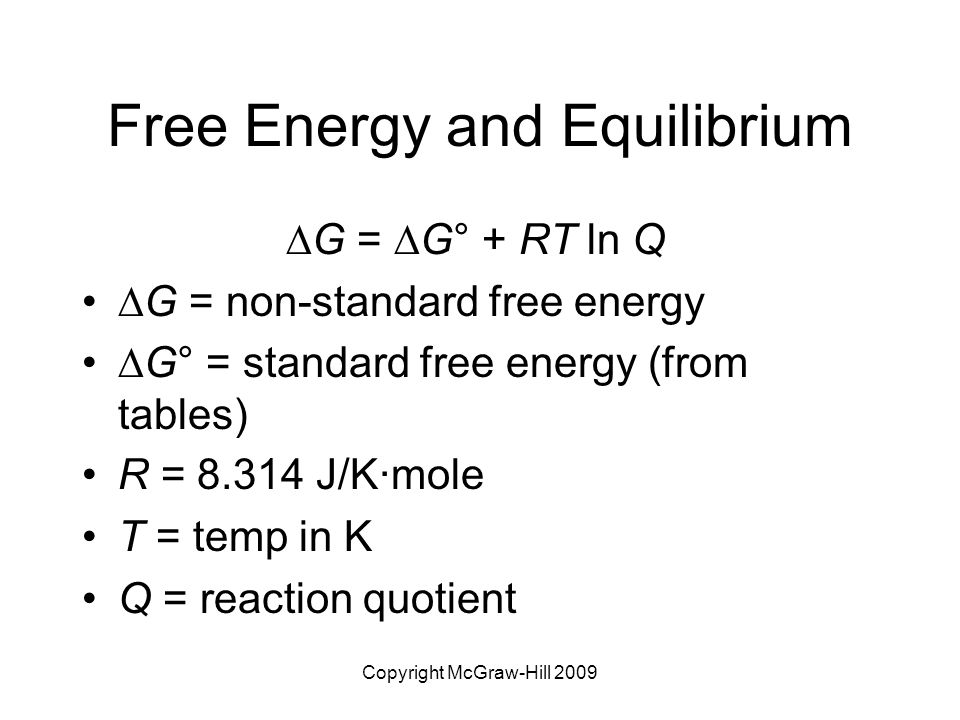
19 6 Gibbs Energy Change And Equilibrium Chemistry Libretexts
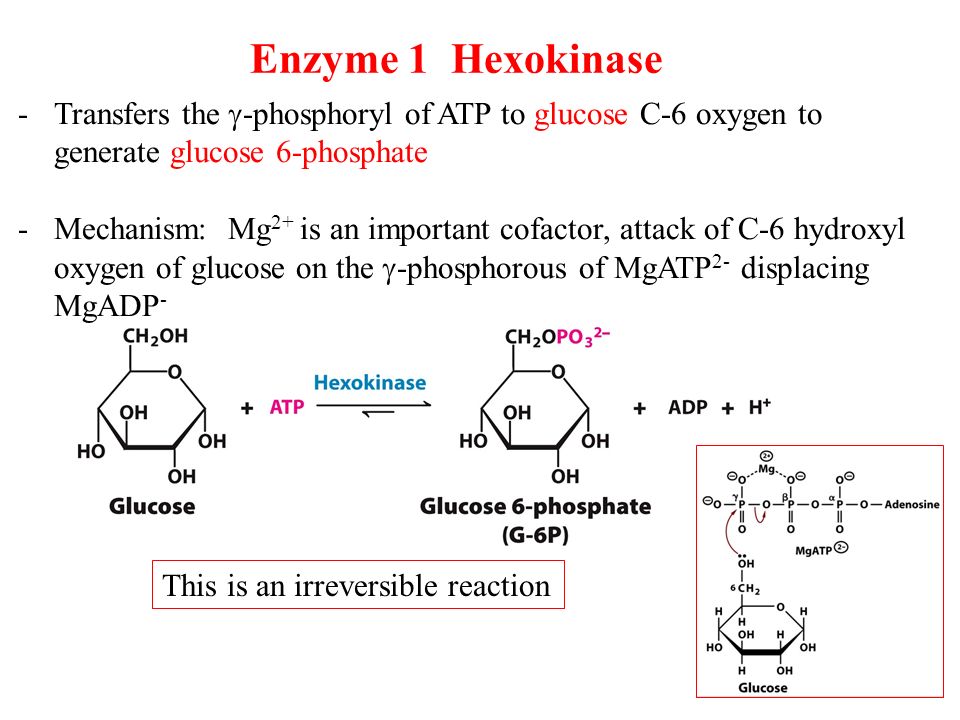
Free Energy Relative Substrate Concentrations And Coupled Reactions In Biochemistry The Credible Hulk
Avoiding First Year University Chemistry Textbooks Misrepresentations In The Teaching Of Spontaneous Reactions
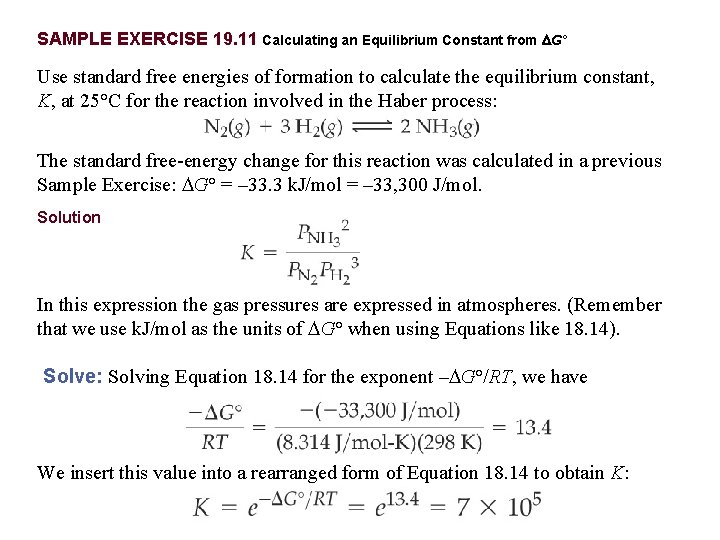
Equilibrium Constant Formula Free Energy
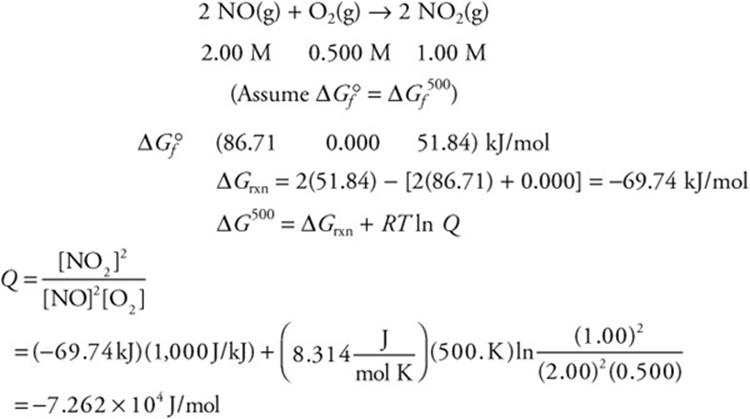
Thermodynamics Review The Knowledge You Need To Score High 5 Steps To A 5 Ap Chemistry 2015
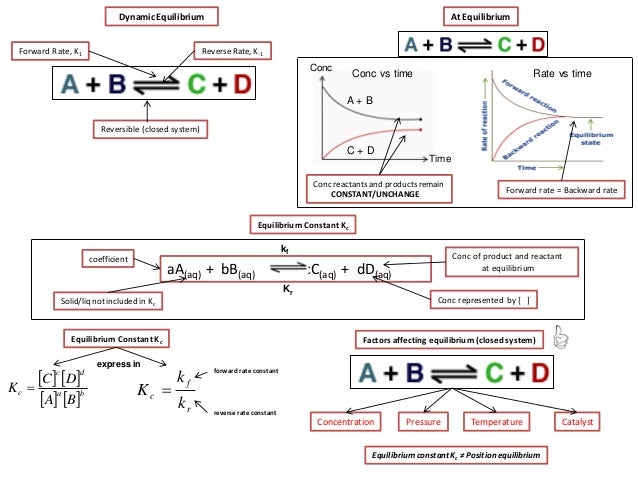
Ib Chemistry On Gibbs Free Energy And Equilibrium Constant Kc

Chm 135 Gibbs Free Energy And Spontaneity W Chemical Potential

Gibbs Free Energy Boundless Chemistry
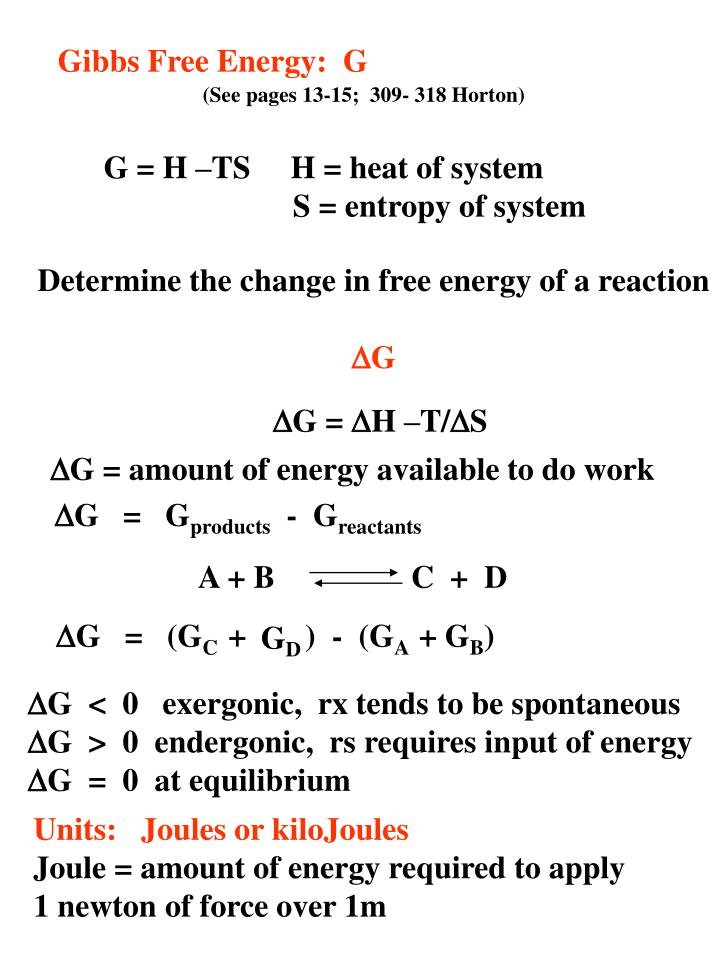
Ppt Gibbs Free Energy G Powerpoint Presentation Free Download Id 3325710

The Standard Free Energy Change Of A Reaction Is Deltag Kj
Gibbs Free Energy And Equilibrium Constant Gibbs Free Energy G Is The Thermodynamic Function That Is Most Useful For Biochemistry G Is A Function Of Ppt Download

17 1 Equilibrium And Gibbs Free Energy Hl Youtube

Cell Potential And Free Energy Protocol

Physical Chemistry Of Polymers Powerpoint Slides
Monster Designs Gibbs Free Energy
Kwok The Chem Teacher Chemical Energetics Applying Gibbs Free Energy
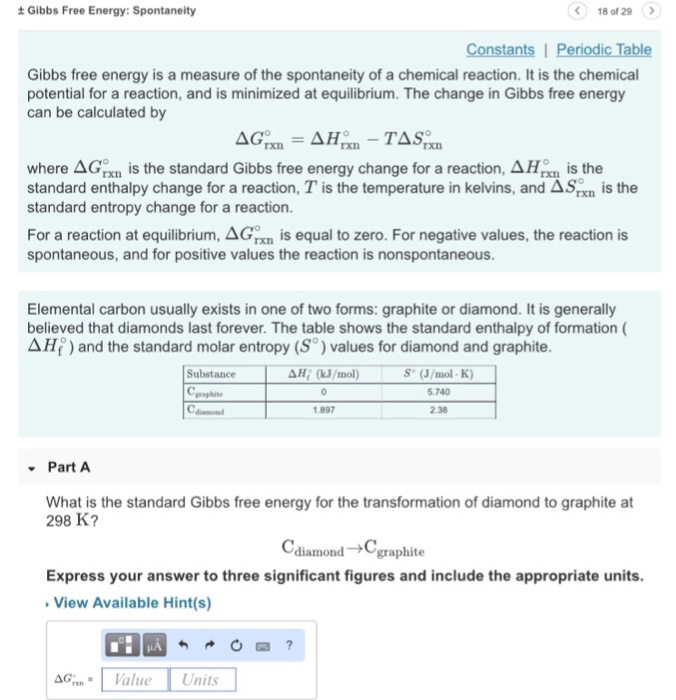
Solved Gibbs Free Energy Spontaneity 18 Of 29 Gibbs Fr Chegg Com

Question Video Standard Free Energy Change For Hydrogenation Of Ethene Nagwa

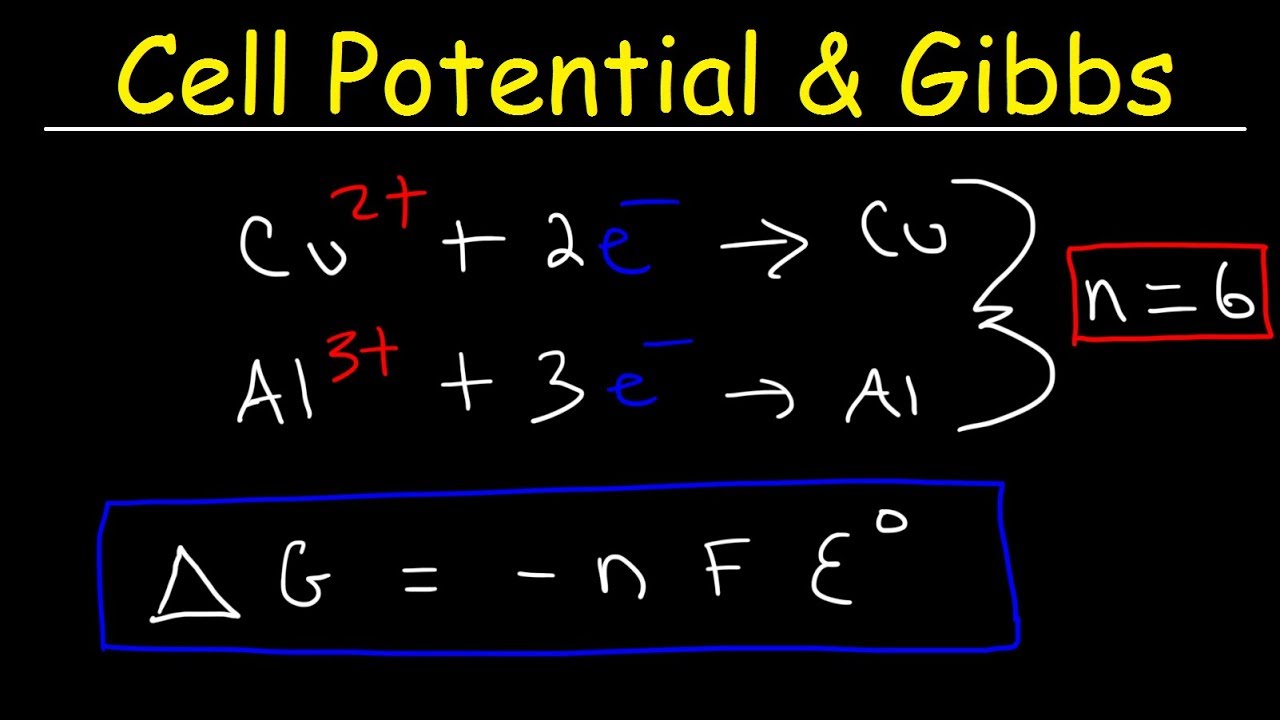
Free Energy And Cell Potential Video Khan Academy

What Will Be Change In Molar Gibbs Free Energy Of H 2 O L At 300 K Constant Temperature If It Is Youtube

Thermodynamics Definition Equations Laws Meaning Formulas Basics Of Thermodynamics
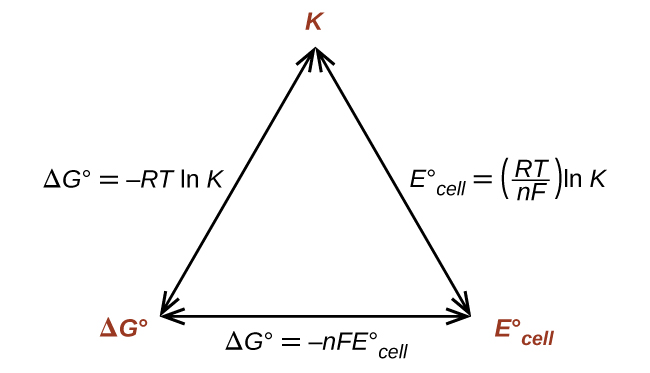
17 4 The Nernst Equation Chemistry

Heat Transfer And Change In Entropy Gibbs Free Energy Ppt Download
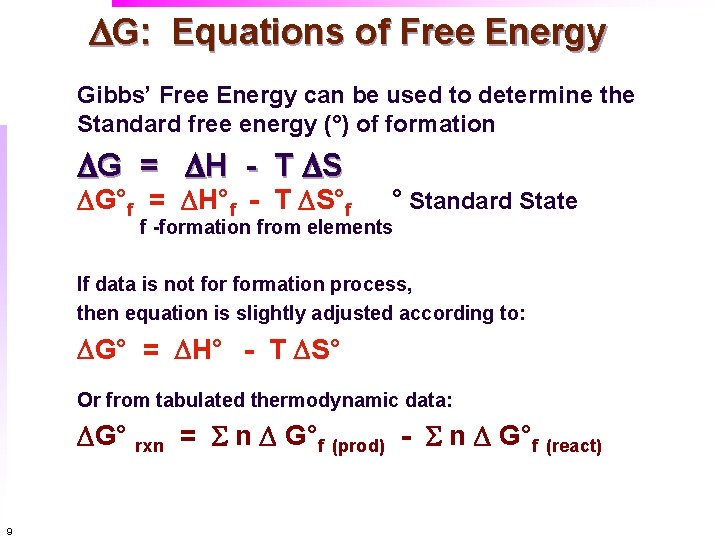
Gibbs Free Energy And Spontaneity And The Meaning

Getting Gibbs Energy As A Function Of Temperature

Pdf The Prediction Of Ph By Gibbs Free Energy Minimization In The Sump Solution Under Loca Condition Of Pwr

Chemical Potential Combining The First And Second Laws For A Closed System Considering Hence For An Open System That Is One That Can Gain Or Lose Mass Ppt Download
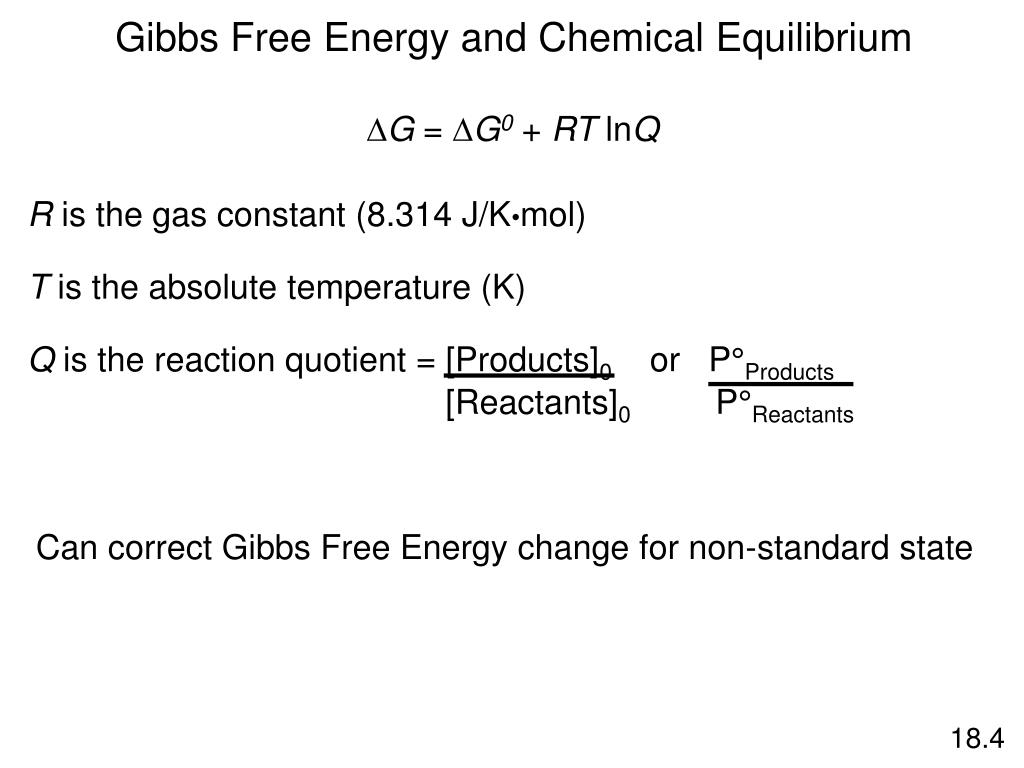
Ppt System Strives For Minimum Free Energy Powerpoint Presentation Free Download Id 4206500

Sandwalk Better Biochemistry The Free Energy Of Atp Hydrolysis
Solved Calculate The Gibbs Free Energy In Kj At 25 Degr Chegg Com

For The Following Reaction The Change In Clutch Prep

How Does Partial Pressure Affect Gibbs Free Energy Socratic

Entropy Spontaneity And Gibbs Free Energy Entropy Entropy

Tang 05 Entropy And Gibb S Free Energy
Solved Gibbs Free Energy G Is A Measure Of The Spontane Chegg Com